Antwort What is Schrodinger equation in chemistry? Weitere Antworten – What is the Schrödinger equation in chemistry
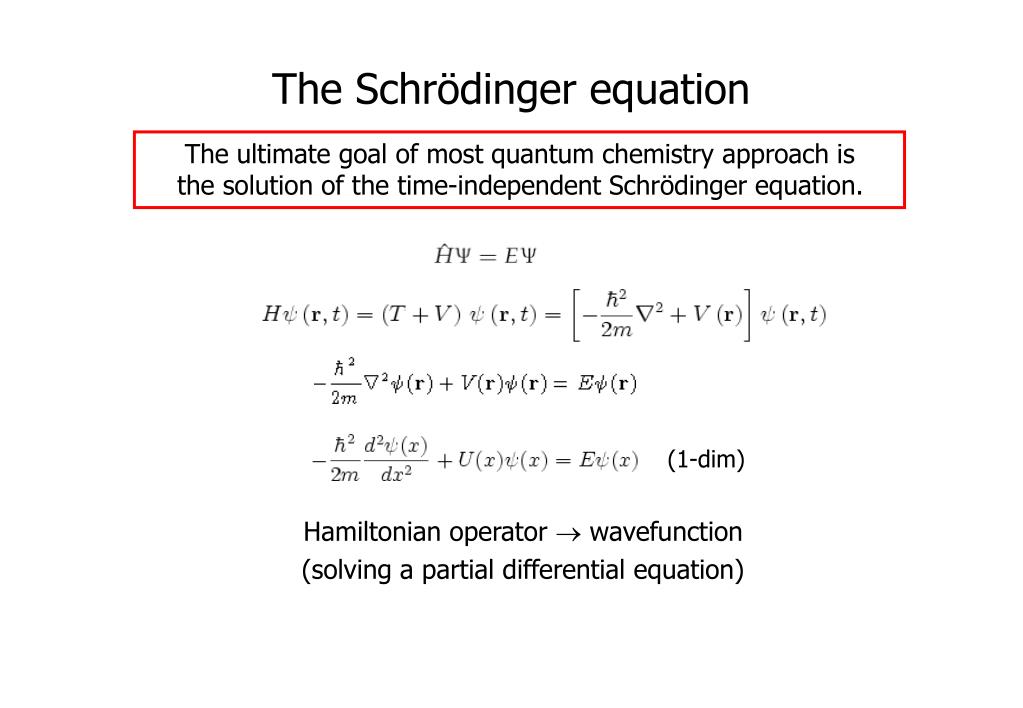
The basic idea of most quantum mechanics methods is to solve the Schrodinger equation ( ( H Ψ = E Ψ ) ) for a system consisting of many electrons and nuclei (a many-body problem). This approach is exact in principle, but simplifying assumptions are generally needed to accurately calculate most systems.The Schrödinger equation gives the evolution over time of a wave function, the quantum-mechanical characterization of an isolated physical system.To generalize this free-particle derivation to a particle in an arbitrary potential, one needs only to add V Ψ V\Psi VΨ to the right hand side. This is sufficient to obtain the result, the time-dependent Schrödinger equation: i ℏ ∂ Ψ ∂ t = − ℏ 2 2 m ∂ 2 Ψ ∂ x 2 + V Ψ .
What is the particle Schrödinger equation : Single-particle Schrodinger Equation
Having determined the wavefunction Ψ ( r , t ) \Psi(\mathbf{r},t) Ψ(r,t), the probability to find the particle at the position r is given by p ( r , t ) = ∣ Ψ ( r , t ) ∣ 2 p(\mathbf{r},t) = |\Psi(\mathbf{r},t)|^2 p(r,t)=∣Ψ(r,t)∣2.
Why is the Schrödinger equation important in chemistry
Schrodinger's equation refers to the state of quantum particles like electrons in an atom, molecule, or solid. This equation accurately describes the properties of the system of electrons in a system.
What does Schrödinger’s equation tell us about electrons : Schrödinger's equation, H ^ ψ = E ψ , can be solved to yield a series of wave function , each of which is associated with an electron binding energy, . The square of the wave function, , represents the probability of finding an electron in a given region within the atom.
The simplest example is that of a constant potential V(x)=V0<E, for which the wave function is ψ(x)=Asin(kx+δ) with δ a constant and k=√(2m/ℏ2)(E−V0).
Unfortunately, the Coulomb repulsion terms (Equation 6.7. 5) make it impossible to find an exact solution to the Schrödinger equation for many-electron atoms and molecules even for two electrons atoms. We have to rely on approximations and the orbital approximation is central to basic chemistry concepts.
What did Schrödinger’s equation prove
Erwin Schrodinger obtained in 1926 an equation that described and explained adequately atomic phenomena and which became the dynamical centerpiece of quantum wave mechanics. The Schrodinger equation yields the eigenfunctions of a particle in an energy potential.The Schrodinger equation is used to find the allowed energy levels of quantum mechanical systems (such as atoms, or transistors). The associated wavefunction gives the probability of finding the particle at a certain position.Unfortunately, the Coulomb repulsion terms (Equation 6.7. 5) make it impossible to find an exact solution to the Schrödinger equation for many-electron atoms and molecules even for two electrons atoms. We have to rely on approximations and the orbital approximation is central to basic chemistry concepts.
(A) It is the bases of wave mechanics. (B) It helps in studying the structure of atom. (C) It shows all the wave like properties of matter.
Why can’t Schrödinger’s equation be solved : Unfortunately, the Coulomb repulsion terms make it impossible to find an exact solution to the Schrödinger equation for many-electron atoms and molecules even if there are only two electrons.
Why is Schrödinger equation hard : Schrodinger's equation cannot be solved exactly for atoms with more than one electron because of the repulsion potential between electrons. You can find more about that in any quantum chemistry textbook.
Is Schrödinger’s equation wrong
The answer obtained in this and the next subsection will be most curious: no, the Schrödinger equation flatly contradicts that the wave function collapses, but yes, it requires that measurement leads to the experimentally observed collapse.
At the beginning of Chapter 3 we have seen that the Schroedinger equation can be solved exactly only for a few physical systems, and we have studied in detail the case of the particle in a box and of the hydrogen-like system.In simple terms, Schrödinger said that if you place a cat and something that could kill the cat (a radioactive atom) in a box and sealed it, you would not know if the cat were dead or alive until you opened the box, so that until the box was opened, the cat was both "dead and alive".
Why do we need Schrödinger’s equation : Significance of Schrodinger Wave Equation
The wave function described by the Schrodinger equation contains all possible information about the quantum system. Solving the equation can determine properties like the probability of finding a particle in a certain location, its energy, momentum, etc.